

Induced pluripotent1 stem cells (iPSCs) are a new type of pluripotent cells that can be obtained by reprogramming any animal or human differentiated cells. They are useful for several reasons: iPSCs bridge the gap between animal models of disease and human clinical trials; they offer an attractive alternative for high throughput screening of novel compounds and can potentially help pharmaceutical companies shortlist lead molecules faster and more reliably; iPSCs are anticipated to create a tremendous impact on modern drug discovery as they enable accurate modeling of human diseases without the ethical concerns associated with extensive animal testing; iPSCs are also extremely useful in the production of allogenic (cells from healthy donors) CAR-T cell2 therapies and address the unmet need for off-the-shelf therapy for patients. Moreover, iPSC-based model systems are beneficial for studying diseases and screening novel drugs where the availability of patient cells is highly limited such as neurological diseases or rare blood disorders.
Syngene scientists have established an in-house capability for generating high quality iPSCs and optimized differentiation protocols to convert them into neural and cardiac cells Figure 1.
iPSCs were derived from peripheral blood mononuclear cell samples of healthy volunteers. These iPSCs were tested for their pluripotency, sterility and absence of viral remnants. Further, these iPSC cells were differentiated either into beating cardiomyocytes or neural stem cells that were further converted into neurons.
Syngene has successfully generated in-house iPSCs Figure 2, ensuring they are not proprietary to any specific client and well-established Standard Operating Procedures (SOPs) ensure the consistent production of high-quality iPSCs. The iPSCs were generated using Good Manufacturing Processes (GMP)-like procedures, to further ensure their quality. Nine-parameter quality control criteria were instigated to ensure high quality. The differentiation protocols were optimized to reprogram iPSCs into various cell lineages, such as immune cells (T cells), cardiomyocytes, neural stem cells, and neurons. This positions Syngene to offer proprietary technology including advantages such as end-to-end service in iPSCs development from design to generation to drug discovery all within a single facility.
1 Pluripotent stem cells have the ability to undergo self-renewal and to give rise to all cells of the tissues of the body.
2 Chimeric antigen receptor (CAR) T-cell therapy is a way to get immune cells called T cells (a type of white blood cell) to fight cancer by changing them in the lab so they can find and destroy cancer cells effectively.
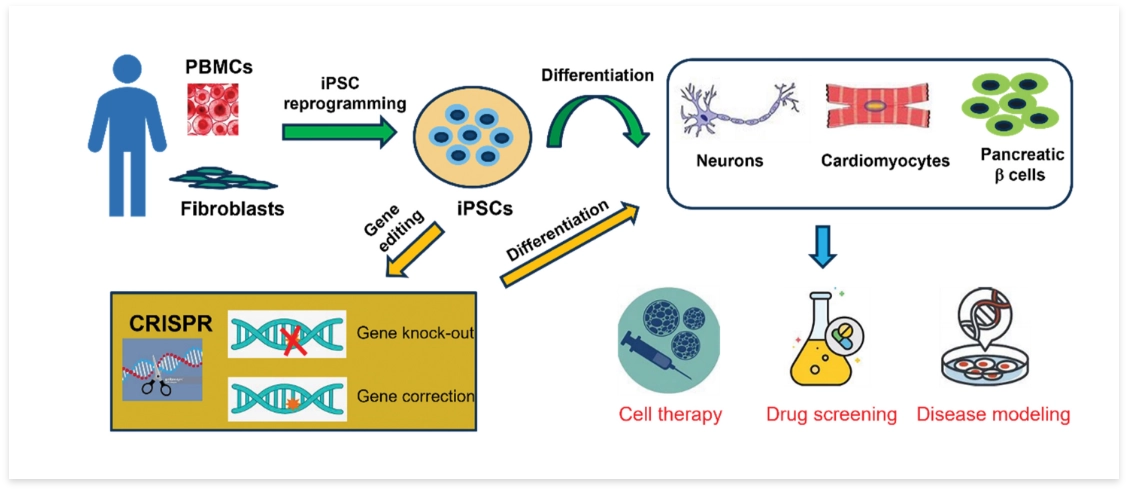
Figure1: Schematic showing the overview of iPSC generation from blood cells and differentiating iPSCs into various cell lineages like neurons, cardiomyocytes etc. iPSCs once generated can be gene edited using CRISPR to correct genetic defects or to make then allogenic for off the shelf therapy. Specific cell types generated from iPSCs can be used for various downstream applications like cell therapy, drug screening and disease modeling.
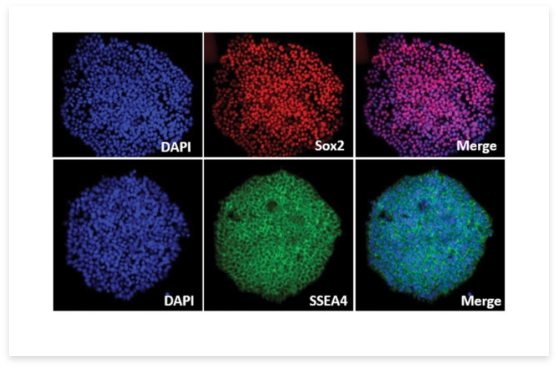
Figure2: Representative images of induced pluripotent stem cells (iPSCs) generated at Syngene and stained with pluripotency markers Sox2 (top panel) and SSEA4 (lower panel) for their characterization.
Rim, Y.A., et al.; J. Vis. Exp. (118), e54650, doi: 10.3791/54650 (2016)
Kadari, A et al.; Stem Cell Rev and Rep (2015) 11:560–569
CytoTune -iPS 2.0 Sendai Reprogramming Kit (USER GUIDE): INVITROGEN
Differentiating Neural Stem Cells into Neurons and Glial Cells: PROTOCOLS, Thermo fisher Scientific