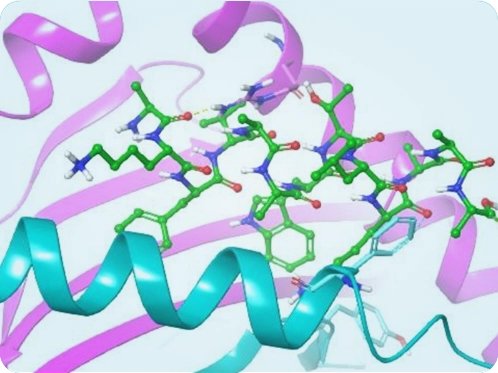
Figure 1 - Peptide docking to MHC Class II allele
The peptide therapeutics market comprises over 80 approved drugs, with many generics introduced upon patent expiration. The production of generics typically introduces additional peptide impurities. These impurities could trigger an undesired immune response, affecting safety and efficacy in patients.
Therefore, immunogenic risk assessment of these impurities in peptide-based drugs is a critical regulatory requirement. This traditionally involves experimental testing, which is time-consuming and expensive. By contrast, in silico assessment (computational models that mimic biological studies) can reduce the need for experimental testing and expedite drug approval.
In silico methods use or learn from experimentally validated or reported immunogenic epitopes1 (from databases) to predict the immunogenic potential of amino acid sequences by:
While these methods generally work with naturally occurring amino acid sequences, they are often ineffective for analyzing sequences containing unnatural amino acids (UAAs).
UAAs may be incorporated into peptide therapeutics to improve the drug product’s properties, or they may be the byproducts of impurities during production.
The Computational and Data Sciences team at Syngene has developed a workflow that can assess the immunogenicity of any amino acid sequence. This is based on modeling the binding of peptide therapeutics featuring UAAs to common HLA alleles (Figure 1). The binding score indicates whether the peptide is likely to be recognized as an epitope and trigger an immune response.
Additionally, the method compares the peptide’s binding score with known immunogens and approved therapeutics to gain a perspective of its relative immunogenic potential (Figure 2).
This method can clearly distinguish between highly immunogenic and safe peptide sequences, as evident from Figure 2, and correlates to experimental results with high accuracy.
Figure 2 – Relative immunogenic potential of the peptide
Syngene’s method provides reliable immunogenicity assessment for any peptide, circumventing the limitation of current methods in sequences containing UAAs. This accelerates drug development, eliminates or reduces the need for experimental safety assessment of generics and facilitates faster regulatory approval.
1It is the precise site on the antigen where the immune system’s receptors attach, triggering an immune response
2HLA proteins are found on most cells and are essential for the immune system. They help distinguish the immune system to recognize “self” versus “non-self” antigens.